Lesson 8: HEAT TREATMENT IV – heat treatment of aluminium alloys
In this lesson, we’ll explore the intriguing world of heat treatment applied to aluminium alloys, uncovering the methods used to enhance their strength and other properties.
- Introduction to Aluminium Alloys
- Aluminium’s advantages include corrosion resistance, low weight, and good formability.
- Its primary drawback is low strength, necessitating heat treatment for improvement.
- Unlike steels, aluminium lacks allotropic transformation possibilities, limiting heat treatment options.
- Strengthening Mechanisms in Aluminium Alloys
- Alloying elements like Cu, Mg, Si, and Zn enhance aluminium’s strength.
- Two strengthening mechanisms: solid solution hardening and precipitation hardening.
- Solid solution hardening distorts aluminium’s crystal lattice, increasing strength.
- Precipitation hardening involves the formation of metallic compound phases that enhance strength.
- Heat Treatment Requirements
- For an aluminium alloy to be heat treatable, several conditions must be met:
- Form a limited solid solution with the alloying element.
- Decrease solubility as temperature drops.
- Form a compound above solubility limit.
- Compound phase must have a sufficiently high diffusion coefficient.
- Composition should be within the range of solubility limit.
- Precipitation Hardening Process
- Precipitation hardening is a complex process consisting of the following stages:
- Solution heat treatment
- Quenching
- Age hardening
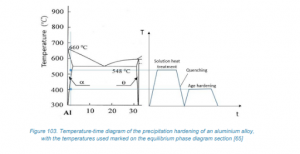
4.1. Solution Heat Treatment
- The alloy is heated above solubility limits but below the eutectic temperature.
- Compound phases dissolve, forming a homogeneous solid solution.
- Avoiding eutectic temperature prevents undesirable partial melting.
4.2. Quenching
- Rapid cooling of the homogeneous solid solution results in an oversaturated state.
- Strength is slightly improved compared to equilibrium state.
4.3. Age Hardening
- The alloy is heated near solubility limits while remaining in the two-phase field.
- Increased temperature accelerates diffusion, leading to compound phase formation.
- Stages of age hardening:
- Formation of Guinier-Preston I (GP-I) zones.
- Transformation of GP-I zones into GP-II zones.
- Appearance of θ’ phase.
- Formation of θ phase.
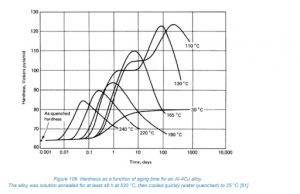
- Optimum time and temperature for aging are critical for maximum hardness.
- Artificial aging accelerates the process, a more economical alternative to natural aging at room temperature.
- Conclusion
- Aluminium alloys offer excellent properties for structural applications.
- Heat treatment enhances their strength, maintaining other favorable characteristics.
- Solid solution hardening and precipitation hardening are key mechanisms for strengthening aluminium alloys.
- Understanding the intricacies of heat treatment for aluminium alloys equips engineers with the knowledge to optimize material performance for various applications.
With this knowledge of heat treatment techniques for aluminium alloys, you can harness the full potential of this versatile material, striking the perfect balance between strength and other desirable properties. This skill is invaluable in various industries where lightweight, corrosion-resistant materials are essential.